
Decontamination for Hospital Pharmacies and Compounding Operations
To ensure the safety and quality of medications, many hospitals choose to carry out their own drug compounding on site. But a drug’s effectiveness and safety can be undermined if contaminants are present during preparation or packaging.
Ecolab’s Bioquell solutions allow you to bio-decontaminate exposed surfaces in your hospital pharmacy, including workstations, cleanrooms, storage areas and pass-throughs. You can also perform drug compounding in an isolator system like the Bioquell Qube, which is an ISO Class 5 environment for aseptic processing, and includes automated bio-decontamination technology.
Why Choose Bioquell
Why should you use Bioquell’s pharmaceutical compounding bio-decontamination solutions?

Bioquell offers containment systems with built-in hydrogen peroxide vapor bio-decontamination, which can be used for both sterile and non-sterile compounding. When preparing hazardous or cytotoxic drugs, the system can be ducted and run at negative pressure.

Whether you’re working with hazardous drugs or sterile compounding, you need to comply with standards such as USP 797 and USP 800. Bioquell offers bio-decontamination solutions that make it easy to comply with regulations. Bio-decontaminate entire rooms and equipment in your pharmacy.
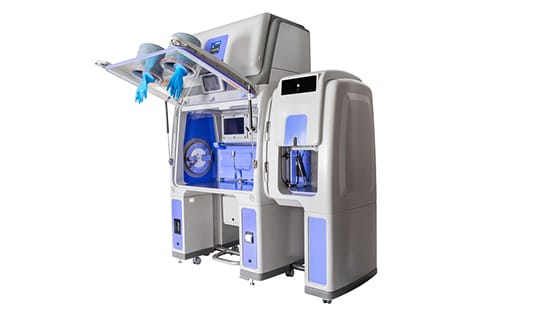
Outsourcing your compounding operations versus performing them internally often means less control over quality, slower turnaround, and higher costs. This is especially true if you process a high volume of medication. With Bioquell's aseptic containment isolator solution, the Bioquell Qube, you can compound drugs on site, to save both time and money.

A drug’s impact can be negated by poor conditions in the pharmacy. Bioquell equipment empowers you to prepare drugs securely, in a bio-decontaminated environment.
Not sure what kind of Bioquell system you need? Let’s talk. We can tailor a bio-decontamination solution to match your pharmacy’s needs.

About Hydrogen Peroxide Vapor
During a bio-decontamination cycle, Bioquell technology fills an enclosed area with 35% hydrogen peroxide vapor. In just one cycle, this vapor provides a 6-log sporicidal kill on exposed surfaces. Unlike chemical sterilants that can leave toxic residues behind, hydrogen peroxide vapor breaks down into water and oxygen once the bio-decontamination cycle and aeration is complete.

Use Even on Sensitive Electronics and Equipment
Our hydrogen peroxide vapor is tough on microbial surface contaminants, but gentle on electronics and equipment. Unlike spray-and-wipe methods, there are no additional steps to remove residue. When you need to bio-decontaminate spaces that contain hard-to-clean equipment, Bioquell is ideal. Before using Bioquell on your electronic equipment, check with the manufacturers to confirm compatibility.
Programs, Products, Equipment and Services
Explore our Biopharmaceutical Manufacturing and Compounding Offerings
We couldn't find any results for "".


